Jelly
Jelly is prepared by boiling the fruit with or without water, adding sugar to a fine sieved extract, and allowing it to set into a clear gel. The jelly should be transparent, well set, but not too hard, and have the original taste of fruit. It should be attractively coloured and with a clean surface that will hold its shape. Pectin is the most essential ingredient in making jelly. Pectin is present in the cell wall of fruits.
As per FPO specifications, good jelly has 65% TSS, 0.5 to 0.75 acid, and 3.1 to 3.3 pH.
Watch Lecture Video
Fruits suitable for jelly are guava, sour apple, plum, Karonda, Wood apple, loquat, papaya, etc. Some fruits have low pectin content, so they are also used for jelly after adding pectin powder. Apricot, Pineapple, Strawberry, Raspberry, etc.
Fruits can be divided into four categories according to their pectin and acid-
- Rich in Pectin and Acid- Crab Apple, Sour Guava, Grapefruit, Lemon, Orange, Sour Plum, Jamun.
- Rich in pectin but low in acid- apple, unripe banana, unripe fig, sour cherry, guava, orange rind.
- Low in pectin and rich in acid- sour apricots, sweet cherries, pineapples, strawberries, and sour peaches.
- Low in pectin and acid- Apricots, peaches, pomegranates, strawberries, raspberries, and other ripe fruits.
Theories of jelly formation
The formation of jelly is due to the precipitate of pectin rather than its expansion. Precipitation of pectin occurs only when pectin, acid, sugar, and water are in a certain balance. The rate of precipitation is affected by the following factors:
- The concentration of Pectin in Solution
- Pectin Formation
- Hydrogen Ion Concentration (pH) of the Pectin Solution
- The concentration of Sugar in the Solution
- Mixture Temperature
There are several theories to explain the formation of jelly.
- Fibril theory: When sugar is added to the pectin solution, it destabilizes the pectin-water equilibrium and the pectin groups form a network of fibrils through the jelly. This network of fibrils holds the sugar solution in the inter-fibril spaces. The strength of the jelly depends on the structure of the fibers, their consistency, and their stiffness.
- Spencer’s theory: Pectin particles are negatively charged. A pectin solution is most stable in the neutral pH range. An increase in acidity or alkalinity decreases its stability. In the manufacture of jelly, sugar acts as a precipitating agent and the presence of acid helps in this.
- Olsen’s Theory: If pectin is considered to be a negatively charged hydrophilic colloid, then the following can be assumed:
- Sugar acts as a dehydrating agent, which upsets the balance between water and pectin.
- Sugar does not dehydrate pectin micelles immediately but requires time to balance.
- If the negative charge on the pectin is reduced with the help of hydrogen ion concentration, the pectin precipitates and accumulates as a fine network of insoluble fibers, provided a sufficient amount of sugar is present.
- As the system reaches equilibrium, the strength of the jelly is maximized.
- Salts and other components that cause changes in the final jelly strength of the system may act by either changing the gelling rate or affecting the final structure of the jelly, or a combination of both.
- Hinton’s theory: This theory is based on the assumption that pectins are complex mixtures of variable composition. According to this, the gelation of pectin is a type of coagulation in which the coagulated particles form a continuous network. Organic acids like pectin dissociate in solution. It is only non-ionized and not ionized pectin, which enters into the formation of the jelly.
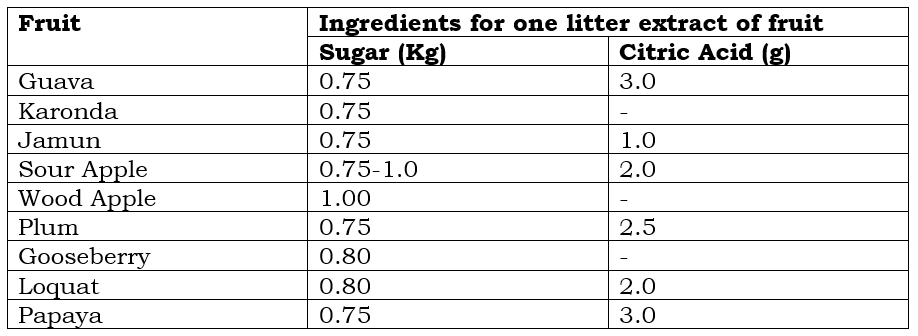
Ingredients
Flowsheet for processing of jelly

Procedure for jelly preparation
(A) Fruit selection: Fruits should be sufficiently ripe, but not overripe, and have a good flavour. Slightly underripe fruits produce more pectin than overripe fruits; As the pectin present during ripening decomposes into pectic acid, which does not form jelly with acid and sugar.
(B) Pectin requirement: Usually 0.5 to 1 percent pectin in the extract is enough to make a good jelly. If the pectin content is high, a firm and hard jelly is formed and if it is low, the jelly may fail to solidify. Pectin, sugar, acid, and water are the four essential components of jelly and should be present in approximately the following proportions: pectin 1 percent, sugar 60 to 65 percent, fruit acid 1 percent, and water 33 to 38 percent. However, the exact proportion of sugar depends on the pectin grade.
Pectin grades: Grades of pectin means the weight of sugar required to set one gram of pectin under suitable conditions to form a satisfactory jelly. For e.g. 100-grade pectin means 100g of sugar is required for the setting of 1 g pectin.
Determination of pectin content
1) Alcohol test- This method involves the precipitation of pectin with alcohol, the outline of which is given below-
One teaspoon of filtered fruit extract is taken into the beaker and cooled and 3 teaspoons of methylated spirit are gently poured from the side of the beaker which is swiveled to mix and left to rest for a few minutes.
a) If the extract is rich in pectin, a single, transparent lump or clot will form. To make jelly in this type of extract, sugar has to be added in equal quantity to the juice.
b) If the extract contains moderate amounts of pectin, the clot will be less firm and fragmented. Three-fourths quantity of sugar has to be added.
c) If the extract is low in pectin, many small granular clots will appear. Only half the quantity of sugar is added.
2) Jelmeter Test – Jelmeter is held in the left hand with the thumb and forefinger. The lower part of the Jelmeter tube is closed with a little finger. The filtered extract is spooned with the right hand into the jelmeter until it is full to the edge. While holding the jelmeter, the little finger is removed from the lower end and the extract is allowed to flow or drip off for exactly one minute, at the end of which closed again with the finger. The level readings in the jelmeter are noted. Sugar is added to the extract as per the readings.
(C) Acid Adding
The jelly formation of the extract depends on the amount of acid and pectin present in the fruit. Of the three acids found in fruits, citric, malic, and tartaric, tartaric acid produces the best results. The total acid in the final jelly should be at least 0.5% but not more than 1% because the acid in excess can cause syneresis.
Jelly pH: The strength of the jelly increases with an increase in pH until it reaches the optimum. The addition of more acid beyond this level reduces the strength of the jelly. The pH of the jelly can be controlled either by adjusting the pH of the pectin extract or by adding an appropriate buffer. The fruits also contain salts like sodium citrate, sodium or potassium nitrate, etc. If the acidity of the extract is high, the finished jelly will stiffer. The optimum pH of the pectin solution is between 3.1 and 3.3 for the best jelly setting.
(D) Sugar adding
It is the essential component of jelly that provides sweetness as well to the body. If the concentration of sugar is high, the jelly holds less water resulting in the jelly becoming hard, probably due to dehydration. Boiled with an acid to invert sugar (sucrose), it is hydrolyzed into dextrose and fructose, the amount of inverted sugar depends on pH and duration of boiling. Due to the partial inversion of sucrose, jelly contains a mixture of sucrose, glucose, and fructose. This mixture is more soluble in water than sucrose alone and therefore the jelly can hold more sugar in the solution without crystallizing.
Sugar in jelly is added according to the pectin test, if pectin is high, then an equal amount of sugar is added, and if there is less pectin, sugar is added only in half the amount.
(E) Determination of end-points: Determination of end-points in jelly can be done using the following methods:
1) Drop Test: A drop of concentrated mass is dropped into a glass full of water. The movement of the drop to the bottom without disintegration (dispersed) indicates the endpoint.
2) Cold plate test: A drop of boiling liquid in the pan is taken and placed on a plate and allowed to cool quickly. If the jelly is ready, the mixture on the plate will shrink when pushed with a finger. The main drawback of this method is that while the drop is cooling on the plate, the jelly mixture continues to boil in the pan and risks overcooking the product or losing the correct setting point.
3) Sheet or flake test: This test is more reliable than the plate test. While boiling the jelly, take out a little jelly in a spoon or wooden spatula and cool it down a bit. After that, it is dropped. If the product falls like a sheet or flakes, it means that the endpoint is reached and the product is considered ready, if it falls as a continuous stream or syrup, it will need to be further cooking.
4) Temperature test: A solution containing 65% TSS boils at 105 °C. Heating the jelly to this temperature will automatically increase the concentration of the solids to 65%. This is the easiest way to figure out the endpoint
(F) Problems with jelly
1) Failure of jellies to set: Sometimes jelly does not set due to the following reasons:
i) Acid or Pectin Deficiency: The jelly may fail to set due to a lack of acid or pectin in the fruit from which it is made. It may also fail to set due to insufficient cooking of the fruit to obtain pectin resulting in insufficient extraction of pectin and acid.
ii) Adding too much sugar: If sugar is added in excess of the required quantity, it results in the formation of syrup or extremely soft jelly. This can be corrected by adding fresh clear juices rich in pectin.
iii) Cooking below the endpoint: If cooking is stopped before the sugar concentration reaches 65 percent, the jelly may fail to set and remain syrupy or overly soft.
iv) Over-cooking: If cooking is continued beyond the endpoint, the jelly hardens due to high concentration. This occurs when both acid and pectin are abundant in the juice and not enough sugar is added. If the acid is high, the pectin breaks down and forms a syrup-like jelly.
2) Syneresis or weeping of jelly: The phenomenon of spontaneous release of fluid from the gel is called syneresis or weeping of jelly. This is due to the following factors:
i) Excess acid: As a result of excess acid, the structure of jelly breaks down due to hydrolysis or decomposition of pectin.
ii) Very low concentration of sugar or soluble solids: This allows the pectin network to hold more fluidity than would be possible under normal conditions.
iii) Insufficient Pectin: This results in the formation of a network of pectin that is not sufficiently dense and hard to hold sugar syrup.
iv) Premature Gelation: Gelation occurs due to the breakdown of pectin during the pouring of jelly into containers. The jelly weakens and breaks.
v) Fermented jellies: Fermentation usually occurs in jellies that have syneresis.
3) Cloudy or foggy jelly: This may be due to the use of the non-clear extract, use of immature fruits (immature fruits contain starch which is insoluble in juice), over-cooking and cooling, non-removal of scum, defective pouring (when the jelly is poured from a great height, the air is trapped in the form of bubbles and the jelly becomes opaque) and premature gelation also causes a cloudy or foggy jelly.
4) Formation of crystals: The addition of extra sugar to jelly can lead to the formation of crystals.
